GLP-1 Weight Loss Drug Shortage: In September 2025, the FDA sent out a record-breaking number of warning letters, in total, to issuers of over 50 companies that sold compounded versions of popular GLP-1 weight loss drugs distributed by blockbuster, including Ozempic and Wegovy. This tough regulatory crackdown came after several months of tension building up after the regulator announced the years long lack of these GLP-1 drugs had been officially ended. Although in October 2024, the FDA removed tirzepatide and semaglutide for the shortage list, in February 2025, a massive parallel market still exists. These drugs are still made by custom-mixing and sold to millions of Americans via telehealth service, medical spas, and online pharmacies.
Table of Contents
Safety Concern Reports About Compound GLP-1 Weight Loss Drugs
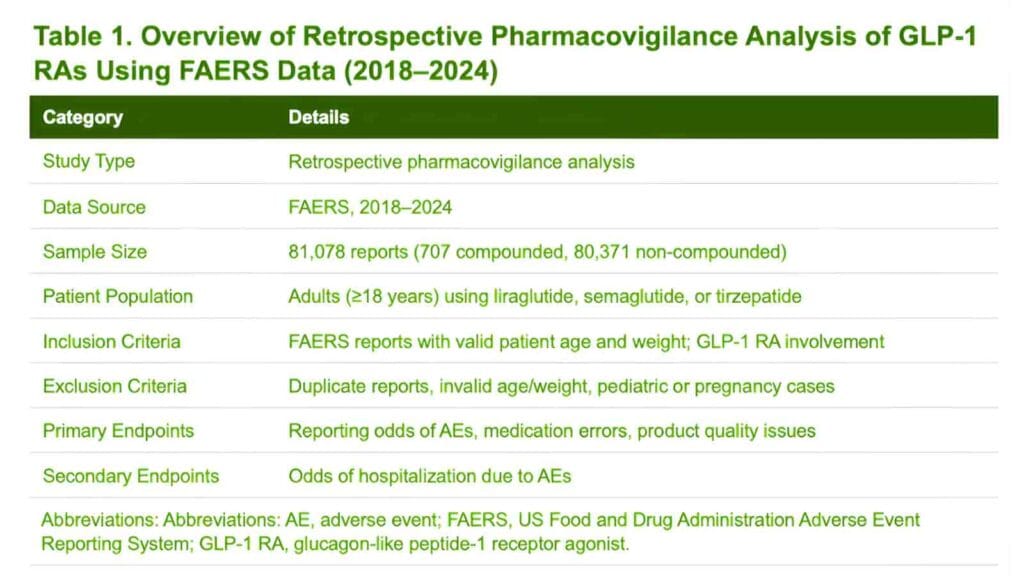
The magnitude of this black drug business is astounding. The compounding market of these particular peptides is projected to grow by 2 billion dollars even though the availability of brand name is restored. This financial flow is accompanied by increasing history of clinical risks. By July 31, 2025, the federal databases had reported 605 adverse event reports associated with compounded semaglutide, and 545 reports associated with compounded tirzepatide. These numbers indicate the huge safety inconsistency between biologics mass-produced and those that were prepared locally.
This change in the regulatory sphere is a significant dilemma to the health of society. The legal loophole under which the shortage had allowed the compounders to circumvent patent protection as it afforded under Section 503A and 503B of the Federal Food, Drug, and Cosmetic Act is theoretically resolved by the resolution of the shortage.
FDA Non-Approved GLP-1 Weight Loss Drug Selling
Nevertheless, a significant number of providers still provide within the grey sectors of the law, taking advantage of the desire of the consumer to get cheaper prices and easier access. This atmosphere creates intense confusing conditions on the part of the patient concerning the legality and sterility of their medication. Where the unregulated versions are continuing to spread, there has never been more importance regarding the differences between a licensed medical treatment and an in potentially dangerous imitation.
The September enforcement measures mean that FDA is planning to seal these gaps, but the size of the current market means that the entire fight of drug safety proponents is going to be a long and bevy of battles.
When the GLP-1 Weight Loss Drug Shortage Started
In early 2022, the world entered a brutal unwarranted shift in the landscape of the metabolic health as the unprecedented imbalance of supply and demand occurred. With a contagious infection of viral social media fads and celebrity profile support, prescriptions of semaglutide have increased their prescriptions by an astounding 442 percent between January 2021 and December 2023. The fact that manufacturers were physically unable to increase production at the rate of the internet-driven demand catapulted the market into an ongoing shortage that lasted until February 2025. This shortage had changed a small drug product into a worldwide cultural mania.
Rise of Weight Loss Drugs Compound Versions
In these years of crisis, a huge secondary market sprung up in the loopholes of the Federal. Since the United States law allows the dating of needed drugs in case of supply deficit, telehealth firms such as Hims and Hers, Ro, and Noom, shifted their business models to service the gap. These sites provided compounded version at a fraction of the price, usually around $200-$300 a month when the brand name injections cost $1000 and above off the list. This pricing gap directed millions of customers to non-branded products, decentralizing the weight-loss business and establishing the infrastructure of hundreds of outlets spread across the country in a haphazard manner.
FDA’s Action To End Weight Loss Drug Shortage
The youth of free compounding came to an absolute turning point at the beginning of 2025. The FDA officially announced manufacturers the ability to satisfy the national demand and introduced a row of set deadlines in terms of strict compliance imposed on the sector. In order to have a smooth transition of the millions of users who use the compounded scripts, the agency had set down certain grace periods: 60 days in the case of traditional pharmacy and 90 days in the case of larger outsourcing plants.
Pharmacies came to a halt in their regulatory clock on April 22, 2025, and their outsourcing facilities were given their last compliance deadline on May 22, 2025. These are the legal dates of the end of the era of shortage. Free market shifted to a chaotic state with the so-called wild west of compounding instead of a highly controlled ecosystem, where the patent owners were the initial owners. This transition made patients and providers to find their way to a new reality of cost increases and increasingly strict distributional channels, and put an end to three years of pharmaceutical improvisation never seen before.
Rise of Pharma Companies Economy By Compound Drugs
FDA grace periods expire mid-2025, which encouraged an unprecedented economic restructuring in the telehealth sphere. The immediately significant drop in revenues created a cliff for the companies that received record-breaking valuations fueled by the development of compounded semaglutide. The lack of the legal safeguards of the shortage, means that there was no emphasis on the quick acquisition of patients and instead on the retention by offering a variety of online products. Telehealth platforms commenced aggressively selling so-called off-ramp initiatives, which redirected users to daily oral prescribed medications or metabolic coaching to counter the reduction in revenue of the compounded GLP-1.
Big Pharma Firms Action After FDA’s Deadline
The brand-name firms, Novo Nordisk and Eli Lilly, acted promptly to regain their share of the market. Pharma multinational corporations increased their patient support packages and set up direct-to-patient delivery channels to match the ease of telehealth startups. This vertical integration enabled the manufacturers to get the high-margin income that was going away to the compounding pharmacies. By the third quarter of 2025 the volume was once again consolidated into these official outlets though the high price point of brand-name drugs still posed a big challenge to uninsured patients.
VCs Investment For Weight Loss Compounding Drugs
This transition also could be observed in investment capital. Investment in the so-called compounding-first startups was slackened down considerably by venture capital, and there was a rush of attention towards the new category of weight-loss therapeutics, including oral small-molecule GLP-1 agonists. These novel medications were easier to manufacture and cheaper in price, which could potentially shake the market without the necessity of such a legal loophole as a shortage. The court litigations between brand manufactures and compounding pharmacies shifted to the legal arena and various high profile litigations have focused on trademark infringement claims and safety claims too.
By the end of 2025 the industry stabilized in a new balance. Clients who had become used to the premium payments of the shortage period had hard decisions to make between the expensive brand names or quit the therapy. This strain gave rise to new national debate of the cost of drugs and patent reforms. The “Shortage That Was” was ultimately a demonstration of concept of the sheer size of the weight-loss market in that the demand to metabolic health intervention is almost price-inelastic when the outcome is ground-breaking.
FDA’s Loophole in Weight Loss Industry
Grace periods of the federal state ended, and the weight-loss industry shifted to the approach of shaping the pharmaceuticals to retain the market share. Telehealth practitioners currently are banking on the personalized formulation exemption clause of the federal law to continue to produce. When these companies are amending the composition of the drugs, they contend that, there is an individual patient benefit realized.
The practice includes the inclusion of additive secondary components such as vitamin B12 or L-Carnitine to the underlying peptide or minor rebalancing of the conventional dosage rates. The leadership of Noom also believes that the compounding law emphatically advocates such degree of personalization in the particular case of the patient.
Legal Debate About This Loopholes
This transition has come under intense scrutiny and debate on the legal legitimacy of the transition.
According to Shweta Kumar of Georgetown Law, it seems these business plans do not comply with the principle of the FDA guidance.
Some manufacturers such as Eli Lilly have gone on the offensive and asserted that any organization that sells mass-compounded forms under pretext of specialized or personalized medicine is presently violating the law.
The battle has shifted to the federal court arena with the Outsourcing Facilities Association initiating two large scale actions against the FDA. According to these filings, the agency has gone early in creativity of the shortage classification through accepting reliance on claims of manufacturer delivery at the cost of real life experience of the providers. According to Ivim Health Taylor Kantor, millions of patients still have to live with such drugs as evidence of shortages in the first line continues.
Affordability of GLP-1 Weight Loss Drugs Vs Compound Drugs
An in-depth affordability divide, propels the ongoing trading of such loopholes. Compound versions are usually under 200 per month, which is a vast difference when compared to the branded products that will cost them 1,000 plus. The majority of insurers will not cover weight-loss symptoms, and the patient will have to make all the expenses on his own. To a good number, the cessation of the official shortage is a financial barrier and not a clinical one.
These patients are then subjected to unwanted substitution with costly brand name medicines which they simply cannot make the full purchase thus leading to a second crisis of treatment availability in the United States.
The legal environment today has become the field of fight between patent protection and access to healthcare. As the FDA tries to revive the regular regulatory norm, the telehealth industry places its hopes on the need to have affordable, modified versions to continue the contemporary metabolic health revolution.
Safety Crisis of Compounded GLP-1 Weight Loss Drugs
FDA Adverse Event Data
The booming growth of the compounded GLP-1 market presented considerable health dangers to the citizens that will be measurable by the middle of 2025. On July 31, 2025, FDA recorded 605 adverse event reports in relation to compounded semaglutide and 545 reports with compounded tirzepatide.
Previous statistics published by December 2024, emphasized on by Senator Jim Banks, already indicated a bleak trend of a total of 900 adverse events and 17 recorded deaths. Such numbers probably reflect only a small part of the actual total since no federal regulation suggests
state-licensed pharmacies to report adverse event, so there might be more underreported events.
Glp-1 Weight Loss Drugs Dosing Issue
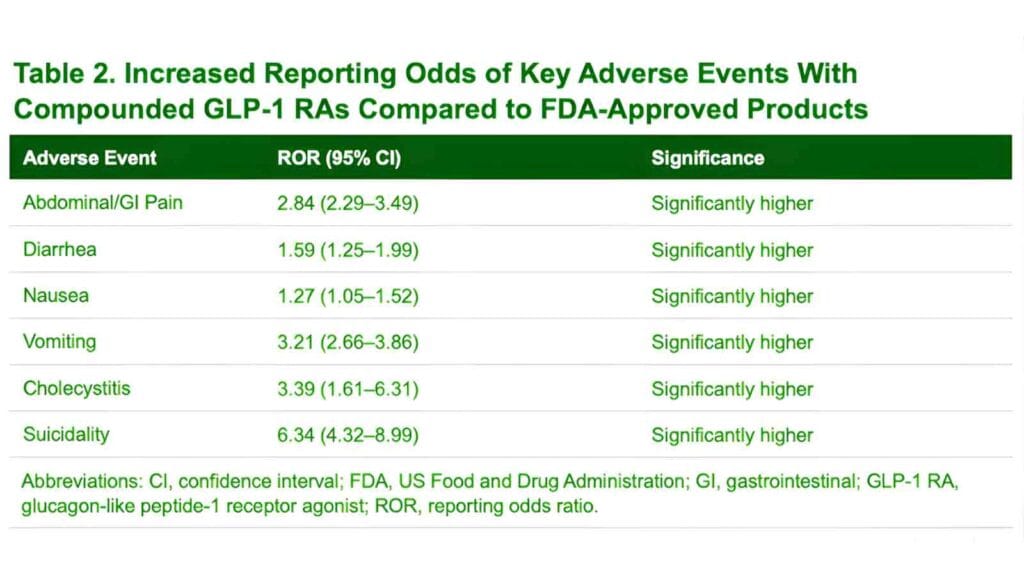
One of the main reasons behind the shortage-induced hospitalizations included the issue of the error of dosing. Patients, as well as health professionals, had a hard time using the new, brand-named pens due to the necessity to use a traditional vial and syringe instead. Absence of experience in the withdrawal of drugs resulted in an epidemic case of over-dosing, and some patients gave themselves up to 20 times the amount of drug they were supposed to receive. This confusion was likely to be because labels were in used to syringe as units and not the standard milligram-per-milliliters concentrations.
The outcomes of such mistakes were unbearable clinical consequences such as acute nausea, unremitting vomiting and life-threatening hypoglycemia.
Compound GLP-1 Weight Loss Drugs Effects
Physiological effects of these non-approved formulations would spread to serious complications that affected the organs. Adverse events documented were acute pancreatitis, gallstones and so severe a case of dehydration that intravenous fluid resuscitation was necessary. In addition to the active compound, the chemical structure of most of the compounded products was doubted both legally and medically. A large number of compounders used semaglutide sodium or semaglutide acetate, salt forms of the drug. The FDA has asserted that these salts are not the same active ingredients as the approved base and has expressed the fact that there is no legal basis to their use in compounding.
Fraudulent of Compound GLP-1 Weight Loss Drugs
Another thing that the crisis contributed to is the boom in fake and fraudulent goods. Investigations found out that there were a lot of vials with labels of the non existing pharmacies or even the pharmacies that really produced the medication but never produced it. The failure of quality control led to another risk to patients since most of the shipments came in improperly refrigerated conditions, meaning that the protein-based peptides were unstable. A dependence on foreign suppliers, in China and India in particular, added to these problems.
It is reported that the Chinese suppliers of API (Active Pharmaceutical Ingredient) took 64% of imports, but some of them had not been inspected, or had been reported to engage in substantial safety infractions.
The FDA‘s Action Against Compound Drugs
In late 2025, the federal government issued broad enforcement onslaught to shatter the illegal market on weight-loss drugs marketing. In September of 2025, the FDA organized a unified warning campaign against over 50 US-based and overseas companies and requested them to take down the false advertisements. This was cautioned against in going to the erroneous assertion that Ozempic or Wegovy compounded versions are generic.
The widespread advertising of these substances based on the same active ingredient with the drug approved by the FDA was also questionable by the agency. The recipients of these letters were under a time limit of 15 days to respond and failure by which the FDA would use forced product seizures or liquidate injunctions against the responders.
What FDA Found Out After The Warning
The crackdown put a lot of emphasis on the issue of digital misrepresentation and the sell of unapproved substances. The regulators found many cases where businesses were saying that their products were undergoing the FDA approval procedures, yet they had never been subjected to the agency and their safety check. This was applied to the sale of retatrutide which is a strong GLP-1/GIP /glucagon triple agonist which is under clinical trials and did not have any medical usage. The promoting of false impression of safety that the terms such as generic Ozempic offered to the unsuspecting consumers is what the prohibition by the FDA aimed to do.
How FDA is Eliminating Compound GLP-1 Weight Loss Drugs
To eliminate the supply of low-quality raw materials, the FDA came up with Import Alert 66-80, or the so-called Green List program. This control mechanism at the border does not allow unverified active pharmaceutical ingredients (APIs) to move into the United States. With such an alert, GLP-1 APIs shipments are automatically detained unless the manufacturing firm has been inspected and approved after review by the FDA. The system ensures there will not be any adulterated or contaminated drug in the domestic drug supply, but the manufacturers who comply and produce quality drugs will not be stopped with this system.
State Authorities Action Against Adulteration of GLP-1 Drugs
These federal efforts to ensure that people are healthy were enhanced by state-level authorities at the same time. Thirty-eight state attorneys general had signing a formal letter requesting the FDA to spearhead a nationwide effort against the hazardous adulteration of GLP-1 drugs. A specialized consumer protection office of the Attorney General of Tennessee provided a certain health notification to the public concerning the upsurge of fraud and the danger of safety of injection provided by med spas or social media. This compound, multi-layered regulatory measure was a useful indicator that the unregulated compounding was coming to a close as the safety and effectiveness that needs to be proven had to be transferred to the sellers as a burden.
What Patients Need to Know About GLP-1 Weight Loss Drugs
The second phase of the post-shortage period requires increased vigilance among the consumers as they pass through the weight-loss drug market.
How to Identify Legitimate vs. Questionable Sources
- The primary prevention against poor quality or fake goods lies in the fact that it is always better to pinpoint the legitimate sources.
- Immediately, patients should stop purchasing vendors that use the term generic ozempic or a purported compounded product is FDA-approved because these are appropriate words that are both illegal and untrue in the medical context.
- The main warning signs are a price that is lower than 100 dollars, the request to pay using a cryptocurrency, or the description of the item as not intended for sale, but rather research.
- The trusted pharmacies should use a valid prescription, deliver drugs in temperature controlled containers, and have their licenses checked by states.
- Confirmation using NABP Safe Site Search tool or a domain of .pharmacy gives a mandatory level of protection.
The Brand-Name Alternative For Weight Loss Medications
Brand-names are now giving more stable long-term treatment options. After the oral Wegovy pill was approved in December 2025, the supplies are now extremely widespread, as of January 2026. Large retailers have also come up with competitive cash only programs to overcome the vacuum caused by compounding. Costco is already offering good-value alternatives at 499 monthly as compared to GoodRx, which is offering vigorous introductory offers at the start-out price of 199 on first two fills. Those programs supported by the manufacturers guarantee that patients get the exact formulation of the chemical, which has been tested during clinical trials and avoids purity concerns of salt-based semaglutide forms such as sodium or acetate.
If You’re Currently on Compounded Semaglutide
A transition strategy should be launched by the patients who are already on the use of compounded scripts. Critical questions during such consultations are to establish the legal status of the pharmacy with the current enforcement of the FDA regulations and to demand some certificates of analysis of the recent batches.
Questions to Ask Your Provider:
- Is this still legal under current FDA rules?
- What’s the source of the active ingredient?
- Are there batch testing results available?
- Can you provide clear dosing instructions in mg/mL?
- What’s the transition plan to FDA-approved versions?
To avoid this, the providers are supposed to provide transparent dosing instructions in mg/mL to avoid mistakes in the administration at the point of switching to branded pens or the new oral tablets. Formal transition plan is used to counter the side effects and continuation of the metabolic benefits realized over the years of shortage.
Insurance Coverage Considerations GLP-1 Drugs
One of the variables that is complicated and will be confusing in 2026 is insurance. Whereas Type 2 diabetes is a normal form of coverage, weight-loss cover differs considerably depending on the employer. In the quest to contribute to employee wellness and retention, more companies are including GLP-1 benefits in their 2026 health plans. When comparing insurance-based brand names to out-of-pocket ones, patients must estimate how much they would spend in total each month on physician check-ups and laboratory work (Seale-Sanfilippo, 2011). Manufacturers patient assistance programs can offer the most sustainable financial course when one has a primary insurance that still does not cover obesity therapies.
The Future Outlook
Market Predictions of GLP-1 Weight Loss Drugs
The metabolic health pharmaceutical environment is being plunged into a phase of enduring structural transformation. Former FDA Commissioner Robert Califf indicates that the termination of official shortages is not an indication of the termination of the compounding industry. According to him, the market is tough with numerous control gambits, and the projected estimated market of the compounding sector of two billion dollar is bound to continue evolving in one or more ways. Numerous pharmacy outlets are already shifting their operations towards drugs such as liraglutide which is still out of stock and new oral preparations who do not depend on the conventional injection-based supply chains.
Pending Legal Decisions
The trend of legal and regulatory pressures will also determine the availability of these treatments in the year 2026. The outcomes of the court cases on the lawsuits of the Outsourcing Facilities Association (OFA) will define the powers of the FDA on shortage declarations. Although enforced mainly by the federal oversight, the state-level regulations are highly inconsistent, producing a patchwork of access throughout the nation. The analysts are foreseeing a consolidation phase where only the most advanced and compliant compounding businesses will still stand, and there may still be an underground market that provides services to those who would like lowest possible prices.
Innovation Pipeline
The technological pipeline provides the alternative route to the stabilization of the market and to the higher competition. Eli Lilly hopes to finish Phase 3 clinical trials of retatrutide, its much-hyped “triple agonist” in early 2026 and an FDA application is likely to follow shortly afterwards. The new generation of “triple G” drugs and oral GLP-1 alternatives will address the goal of offering enhanced efficacy and simpler production than in the current generation of peptides. Such an influx of new products can eventually bring price competition to the prices of the general population, but patient education is essential so that people will be able to differentiate between regulated breakthroughs and untested alternatives.
Conclusion
The formal solution of the GLP-1 deficiency signifies a transition into a more difficult regulative and clinical stage. Whereas the semaglutide and tirzepatide supply chains have become stable, the market is now overrun with compounded versions that have recorded safety concerns. The FDA is also executing vigorous compliance operations against plants that indulge in improper marketing online or manufacture of low quality products. The existence of the legitimate compounding is now limited to restricted medical exceptions of individual patients, and not the mass-market production that occurred during the years of crisis.
Key Takeaways:
- Shortages officially over, but controversy continues
- Compounded versions carry real safety risks
- FDA actively enforcing against deceptive practices
- Legal compounding exists in narrow circumstances
- More affordable FDA-approved options emerging
In 2026 there will be a greater choice of affordable, FDA-approved options in metabolic health due to the existing pharmaceutical landscape of 2026. The patients now find themselves at a crossroad between brand-name therapeutics and unregulated compounds when choosing the option of lower costs. New insurance cover requirements and the influx of generic versions are increasing access to legitimate medicine.
The paramount standards that any long-term treatment plan should be gauged by are safety and legality. Immediately, patients are to check the validity of their existing prescriptions and discuss those that are recently available on the market as the FDA-approved alternatives with healthcare providers. Reporting any negative incidences under FDA MedWatch system will guarantee constant safety of people, as well as assist the regulators in enforcing the changing market. The most critical aspect that one should be conscious of in this post-shortage period is to be aware of these changing regulations so that one can adhere to the metabolic health regimen.

