Reverse Aging: The future of geroscience especially in the year 2025 is one that has manifested a significant increase in the sphere of organizing significant evidence regarding the idea of aging being a treatable process. There have been four major breakthroughs, which have offered a new approach of intervention.
Read About: Inside Jeffrey Epstein’s New York Home | Hidden Cameras and Billionaires
photos Revealed in 2025The acceptance of aging as an irrevocable continuous process of dwindling has been part of human history in most life times. The current medical paradigm has concentrated on the idea of one disease at a time in that the symptoms of age-related diseases, such as heart disease and Alzheimer disease have been regarded as separate and distinct pathological events.
Nonetheless, the paradigm of science is changing fast. The Geroscience Hypothesis supposes that through directing efforts against the basic biological aging pathways, one can postpone, forestall, or resolve a broad range of age-related diseases in a concurrent manner as opposed to addressing them sequentially. The new form is aimed at discussing the cause behind the morbidity in the elderly rather than its symptoms.
Table of Contents
New Discoveries on Reverse Aging
Over the past year, 2025, the findings have followed one after another which are rendering this hypothesis as a medically viable plan. The discoveries provided the first concrete, multidimensional scientific evidence that the process of human aging is much more bendable than ever considered before. Irreversible biological age measurement and the active reversal of cellular and genetic age-related phenotypes are coupled with an appreciation of the complexity and organ-specificity of aging, which have been combined into a single intervention model. This presents the nexus of events that shows that 2025 was the year the promise of the medical anti-aging moved to the realm of reality.
A Molecular Primer of the Foundational Science of Aging
Before discussing the appreciation on the relevance of recent discoveries, one should first know the mechanisms underlying the aging process. The damage accrued to cells and their molecular machinery during the course of a lifetime is well established to be a key driver of aging and age-associated disease. In this section, one will describe the three important biological markers that are the objective of the new wave of anti-aging interventions.
Cellular Senescence: the Zombie Cell Phenomenon
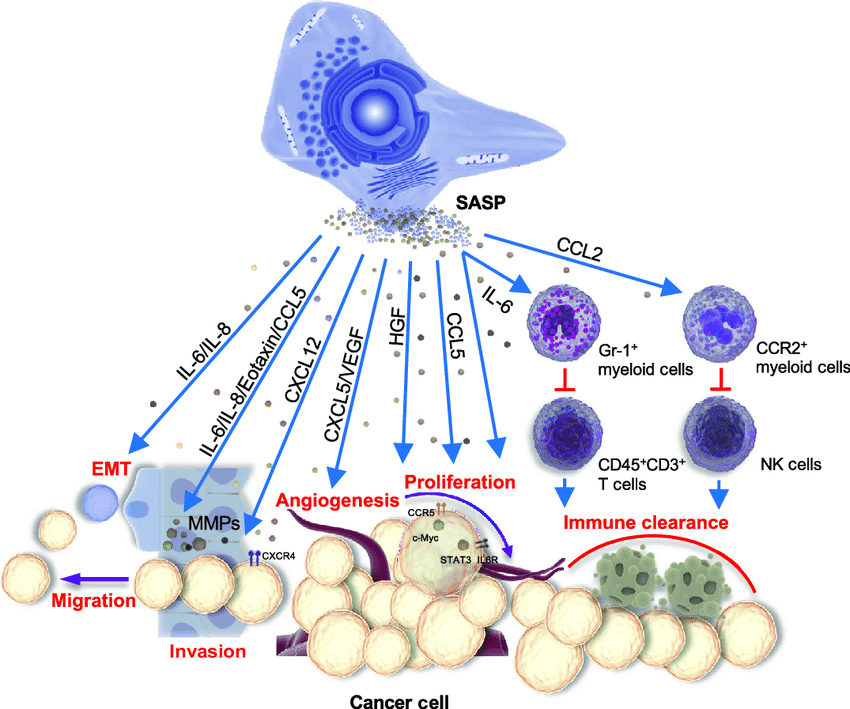
Cellular senescence refers to a non-proliferative arrest of the cell cycle, enacted in response to numerous endogenous and exogenous stimuli, including karyotypic entry, or oncogenic stress. Senescence can protect by stopping the growth of bad or aged cells and inhibiting the growth of potentially cancerous cells. Nevertheless, they can be metabolically active but the main task of performing various tasks according to the character of the tissues they are becomes inhibited or rather paralyzed and hence they are usually referred to as zombie cells.
This tendency makes the cells accumulate in different tissues as we grow because the consumption capacity of the immune system decreases.An important regulation through which senescent cells promote aging is called the Senescence-Associated Secretory Phenotype (SASP). These multifactorial mixtures of mainly pro-inflammatory products of senescent cells form what is called a toxic microenvironment which undermines tissue repair, regeneration and functional status. The SASP contributes to the cause of age-related illnesses, including organ dysfunction and chronic disease, and is a key contributor to chronic inflammaging, the age-related chronic inflammation syndrome.
Telomere Attrition: A Clock Count Down of the Body
The protective caps found at the terminals of the chromosomes, which are important in the maintenance of the genomic integrity, are called telomeres. Some of the telomeric DNA is lost during the division of the cell in the process referred to as telomere shortening. At a certain critical length, short telomeres trigger the replicative senescence or programmed cell death (apoptosis) of a cell. Telomere length is, therefore, a kind of biological clock that dictates the limit of proliferation of somatic cells, which is a definitive signal to the age of cells.
This shortening can be blocked by the addition of repeats of the sequence TTAGGG to the ends of chromosomes by an enzyme called telomerase, but this process is mostly confined to germline and stem cells, and in most normal somatic cells that enzyme is dormant. The degeneration of these cells through loss of telomeres causes their entry into senescent state, which affects the health and longevity of a person.
The DNA Damage Theory of Aging: An Essential weak point
DNA damage theory of aging suggests that unrepaired injuries in the genome DNA is one of the main underlying causes of the aging process. This harm occurs due to a variety of causes and may change the DNA sequence or structure, such as environmental stressor pollution, metabolic bi-products such as reactive oxygen species (ROS), and damage that can occur during the process of DNA replication and repair.
Oxidative DNA damage, e.g., 8-OHdG, has been seen to accumulate in the brains, hearts and skeletal muscles of aging humans and animals. This gradual wear and tear on the genetic blueprint highlights the urgent necessity to do something that can prevent this erosion or restore it.
Breakthrough 1: Reversal Epigenetic Age

The really compelling thing about 2025 is the real demonstration of the reversal of aging, potentially at the molecular level as quantified by the gold standard in longevity biomarkers: The epigenetic clock.
The Horvath Clock: A novel Aging Biomarker
It is a highly accurate estimate of a person biological age measured based on the Horvath epigenetic clock, a biochemical test. Dr. Steve Horvath developed the clock, which is the measure of the level of DNA methylation, which involves the addition of methyl groups to DNA molecules and controlling the expression of genes. The groundbreaking aspect of the clock is that it is highly generalizable and applicable to a vast variety of human tissue and cell types, as all of the 353 different epigenetic markers are used in the clock. This generality gives it strength and objectivity tool to estimate the impact of anti-aging treatment having a very good correlation coefficient of r = 0.96 with real age.
Epigenetic Rejuvenation and Senolytics
In one key study of the year 2025, it was found out that some senolytic agents can directly regulate this biological clock. Human blood was exposed to eight senolytic compounds in an in vitro experiment over a period of three days in a controlled experiment.
Breakthrough 2: Cellular Rejuvenation Controlled By Genetics
At the level beyond the epigenetic level, 2025 saw a breakthrough in studying aging at the cell level that is the most fundamental unit. This study claims that aging can be seen as an activated state which can be switched off at the genetic level rather than as a passive condition to be countered.
The New Anti-Aging Target- AP2A1
Osaka University conducted a pioneer study that established a major protein subunit, AP2A1, as a determinant of switching between the cell state of being young and old. The research concluded that there is increased expression of AP2A1 in the stress filaments of senescent cells such as fibroblasts that help the skin to retain its structural properties.
To see what it does, researchers performed a key experiment: they knocked down the expression of AP2A1 in aging, senescent cells and, in contrast, over-expressed it in younger cells. The implications were quite dramatic: down-regulation of the protein in older cells caused the cells to “revert senescence and stimulated cellular rejuvenation” and the up-regulation of the protein in younger cells brought about “accelerated senescence”.
Mechanism of Action: Structural Basis of Aging
This finding gave a new meaning to a previously well-noticed observation: enlargement of size of senescent cells. The study has also found the AP2A1 to operate very closely with another protein.
integrin β1. The two proteins travel through the swollen stress fibers in the senescence cells, strengthening the attachment of the cells to their extracellular matrix. This process ensures a structural reasoning in why such old cells will be able to sustain their unique large and in many cases thickened bodies. The targeted AP2A1 allowed the researchers to find that it was a method of preventing this structural strengthening that would enable the cell to revert back to a more youthful appearance and functioning.
The finding that one protein can serve as a “toggle switch” to induce cellular rejuvenation alters the conceptual landscape of aging basicly. It takes the field in a new direction past just clearing or repairing to one with the possibility to reprogram cells to a younger state.
Breakthrough 3: The New Map of Aging: Organs Don t Age Alike
Uniform, systemic, decline has been the conventional representation of aging. But a new study has shattered this notion and as a result of the research it has been realized that organs of the same individual have varied rates of maturing and that this heterogeneity contains important clues to developing disease.
A Proteomic Aging Atlas A “Proteomic Aging Atlas”
An innovative study headed by a team of researchers at University College London (UCL) and provided in The Lancet Digital Health disclosed that individual organs of a person can be biologically age-determined through an easy blood test. Studying thousands of proteins in a single blood sample, researchers generated a so-called proteomic aging atlas that plots the unique aging signatures of organs including heart, lungs, kidneys, and brain.
The essence of the conclusion is that something researchers call the age gap between an organ and the chronological age of the bearer is a strong predictor of the subsequent disease risk.
Clinical Significance: Early Prediction of the Disease
A strong correlation was found: a fast-growing organ age gap indicated the danger of 30 various ailments in the other half of 20 years in initially healthy individuals. Such a faster aging heart (an example) indicated a considerably higher risk of developing cardiovascular diseases. Likewise, lung aging, which was accelerated, predisposed people to respiratory infections, COPD and lung cancer. Discoveries also led to unexpected links like the fact that the greatest risk of dementia was not the aging brain but the typically accelerating immune system.
As the study co-author Professor Tony Wyss-Coray explained the finding proves that there exists an absolutely important causal relationship an old age in one of the organs can cause the other organs to fail. This gives a strong answer to the question that the disease tends to be represented as comorbidities of age-related diseases. Aging is not a set of isolated breakdowns but an interconnected system in which malfunction of one component can lead to cascade of malfunction in other components.
| Organ with Accelerated Aging | Associated Disease Risks |
| Heart | Cardiovascular diseases |
| Lungs | Respiratory infections, COPD, Lung cancer |
| Immune System | Dementia, Agranulocytosis, Lymphatic node metastasis |
| Kidney | Vascular disease, Type 2 diabetes, Liver diseases |
Breakthrough 4: Future of medicine: Combination Therapy
The hope of getting at one particular aging process may be fading in all favor of a more advanced, multi-drug solution. A groundbreaking 2025 study on mice has given us the strongest evidence to date that combination therapy will be the way forward in areas of anti-aging medicine.
A Mouse Epic
In a paper published in Nature Aging, researchers headed by those at UCL and the Max Planck Institute for Biology of Ageing showed that mice have lifespans dramatically extended when two approved cancer drugs enthusiasts include-Micrograms and Trametinib used. Although the use of a single agent, Rapamycin, which is a well known geroprotectant, or Trametinib increased lifespan increase by 15-20% or 5-10% respectively, the combination treatment achieved a phenomenal lifespan increase in about 30%.
In addition to the quantitative extension of life, the combination therapy doled out significant health gains also. The treated mice manifested reduced chronic inflammation of any tissues and brain and late development of cancerous tumor at old age.
Principle of Synergy
This was not a mere case of additive effect of this drug combination. The analysis of gene activity in different tissues by the study indicated that Rapamycin and Trametinib combination had an impact that could not be achieved when the medicines were taken alone. This is a pointer that there was a synergistic effect of the two compounds which unlocked new anti-aging properties, which are not possible to achieve by a single drug.
This research is a strong indication of the concept of synergy which is already gaining momentum due to technology.
A decisive change in the approach to therapy is highlighted by the success of the Rapamycin and Trametinib combination. Rather than single, monolithic targets, longevity medicine of the future will rest on systems-level knowledge of aging, and needs to target synergistic combinations of pathways (drug cocktails) based on personalized, combined parameters.
The Moral and Populace intersection
Amid increasing scientific advances toward making the reality close to the promise of anti-aging medicine, a complicated issue of ethics and social questions are taking center stage. The possibility of expanding human health and life undermined traditional values dearly rooted in our society and poses serious concerns on the issue of access and equity.
Lifespan vs. Healthspan
One of the most valuable discussions that can be made in philosophy concerns the central aim of anti-aging interventions: do we want to live longer in years (lifespan) or longer with health (healthspan)? We should make those years have new life as John F. Kennedy once said, and not add new years to life. The healthspan is being explicitly put as a priority by such major institutions as the Mayo Clinic and the Global Roadmap for Healthy Longevity Initiative, being the more egalitarian and healthful of all aims.
The issue of Inequality and Access
Of great concern is the risk that anti-aging medicine may become a luxury applied only to the rich classes hence worsening the health inequalities. The situation of health disparities is already too well entrenched, as social factors enacting the disparities include income, education, and access to quality healthcare services. With the advent of new, expensive modalities, there may be two levels, the privileged who can afford to postpone the manifestations of aging, and the rest of us who are still just as susceptible to the same diseases and do not escape age related decline.
The problem of the inequality does not lie only in the access to the money, but in the prejudices inside the society. The controversy regarding the types of people who are supposed to end up receiving anti-aging interventions inseparably relates to the matter of ageism. Ageism is a systematic bias that even today creates care rationing and lower quality of care to the elderly, as is documented in one of the theme issues of AMA Journal of Ethics in 2025.
The Road Ahead:lab-to-clinic and Beyond
The 2025 breakthroughs represent a giant leap out of the theoretical and into the practical, but the pathway out of the test lab into mainstream clinical use is only starting.
Present Stand of Human Trials
The outcomes of human experiments with senolytic drugs are complicated and not yet clear. Phase 1 pilot study of senolytic combination therapy Dasatinib plus Quercetin (D+Q) in patients with diabetic kidney disease has been successful by showing reduction in the burden of senescent cells in adipose tissue, and skin.
The research showed that even a small treatment, or a hit-and-run treatment, in which a person took a three-day regimen of oral medication, was enough to reduce the burden of senescent cells days-to-weeks later, indicating that the treatment was effective. An additional small Phase 2 study of D+Q in an attempt to benefit bone health demonstrated only modest clinical improvements, however.
The mixed findings underline the difficulty in converting senolytic activity to where it matters, i.e., to clinically relevant outcomes, and the necessity to develop more research before the correct way to apply these therapies is understood.
Conclusion: Future of Aging might Have Already Arrived
It is highly likely that the year 2025 will be considered as the time when the scientific community obtained a deeper and different knowledge about the process of aging. The coincident of breakthroughs, the ability to reverse epigenetic age and modulate cellular senescence genetically, the mapping of organ-specific senescence and the strong effect of multi-drug combinations, prove that aging is not the infinity of vertex but is subject to intercession by the use of drugs in a multi-drug combination.
The revolution will not only end up extending years to lives but also lives to years. It poses the final question and one that everybody must ask himself or herself about this new age of possibility: Would you live to age 120 in perfect health?
Read More Here.

